|
|
| Symbol: |
Qkiqk-v |
| Name: |
quaking, KH domain containing RNA binding; quaking viable |
| MGI ID: |
MGI:1856539 |
| Synonyms: |
qk, Qkqk, qkv |
| Gene: |
Qki Location: Chr17:10425480-10538706 bp, - strand Genetic Position: Chr17, 7.75 cM
|
| Alliance: |
Qkiqk-v page
|
|
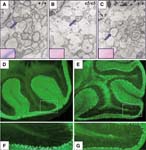
Brain morphology in Qkie5/Qkie5 and Qkiqk-v/Qkiqk-v mice
Show the 1 phenotype image(s) involving this allele.
|
|
|
|
|
| Allele Type: |
|
Spontaneous |
| Mutation: |
|
Deletion
|
|
|
Qkiqk-v involves 7 genes/genome features (Qki, Cahm, 1700110C19Rik ...)
View all
|
| |
|
Mutation details: The quaking phenotype has been attributed to a 1.85 Mb deletion on chromosome 17. The proximal breakpoint was located in the promoter region of the Qk gene and affects transcript levels of that gene. The distal breakpoint lies between exons 5 and 6 of the parkin gene. Both the parkin gene and another co-regulated gene, Pacrg, are inactivated. Although parkin is not expressed in these mutants, the described phenotype appears due to to the defect in Qk expression.
(J:55007, J:87498, J:88351, J:90667, J:101474)
|
| Inheritance: |
|
Recessive |
|
|
View phenotypes and curated references for all genotypes (concatenated display).
|
|
|
|
List all tumor models in MMHCdb carrying
Qkiqk-v
|
|
|
|
|
The quaking mutation arose spontaneously in the DBA/2J strain. Homozygotes have marked rapid tremor which disappears when they are at rest but increases during locomotion. It begins at about 10 days and is fully developed by 3 weeks. Mature mice may have seizures in which a motionless posture is maintained for many seconds. Females are viable and fertile, males sterile.
Homozygotes are severely deficient in myelin, the material which ensheathes and insulates the axons of the central (CNS) and peripheral (PNS) nervous systems (see Mbp). The entire CNS is very deficient in myelin at all ages (J:13141), and there is a less severe myelin deficiency in the PNS nervous system (J:5177). Myelin sheaths are present in the CNS, but they are thinner than normal, some consisting of only one to four myelin lamellae. The sheaths are usually loosely wound, with patches of oligodendroglial cell cytoplasm between the lamellae, and there are abnormal inclusions and vacuoles in the processes and perikarya of oligodendrocytes. Development of the myelin sheaths appears to be arrested in a stage characteristic of very young animals (J:5189)(J:5271)(J:5218). There is variable hyperplasia of oligodendrocytes, greatest in the tracts with the greatest degree of myelination (J:5615). Axons have normal morphology but there is abnormally high proteolysis in the axons of the optic nerve (J:6971). There is evidence that the myelination defect in the CNS is due to defective oligodendrocytes (J:6216).
Handling-induced convulsive seizures in qk/qk mice can be inhibited by administration of N-methyl-D-aspartate (NMDA) antagonists. Modulatory mechanisms for the NMDA receptor complex may differ in qk/qk mice from wild-type (J:1930). a2-adrenoceptor (A2A) antagonists also inhibit these seizures, while A2A agonists potentiate them. qk/qk mice have increased brain binding sites for A2A agonists (J:1169).
In the PNS, thinly myelinated and unmyelinated fibers have been described in the sciatic nerve and in the intracranial portion of the trigeminal nerve (J:5189)(J:5271). The sheaths may be structurally abnormal with regions of uncompacted myelin lamellae similar to those of the CNS (J:5778). Orthotopic transplantation of pieces of sciatic nerve between quaking and normal mice has shown that the genetic defect is expressed in Schwann cells (J:14892). Qk causes defective myelinogenesis in both oligodendrocytes and Schwann cells (J:6411).
There is an extensive literature on biochemical defects related to the deficiency of myelin in quaking mice (J:26986), a consistent finding of which is a severe deficiency of the myelin lipids, sphingomyelin, cerebrosides, and sulfatides, particularly those containing long-chain fatty acids. The normal increase in these fatty acids which occurs between 15 and 20 days does not occur in qk/qk mice, so that adult mutants tend to resemble very young controls (J:5171). Brain proteolipids in adult quaking mice retain the relative proportions found in 10-day controls (J:5408). The myelin-associated glycoproteins of different molecular weight in the brains of quaking mice 15 days of age and older are expressed in abnormal proportions (J:7990). Synthesis of myelin basic protein and proteolipids is normal in quaking brains but their incorporation into myelin is defective (J:6151). mRNAs for myelin basic protein, for example, occur in oligodendrocyte cell bodies, but not in the cell processes that actually form the myelin sheath, in qk/qk brain (J:1931). Quaking mice may have abnormal levels of copper and zinc in the brain, but the evidence on this point is conflicting (J:7214).
The sterility of male qk/qk mice is due to defective spermatid differentiation, the details of which have been described (J:5241). It has been further demonstrated that male sterility in these mice is the result of the loss of Pacrg expression (J:90667).
|
| Original: |
J:13141 SIDMAN RL, et al., MUTANT MICE (QUAKING AND JIMPY) WITH DEFICIENT MYELINATION IN THE CENTRAL NERVOUS SYSTEM. Science. 1964 Apr 17;144:309-11 |
| All: |
96 reference(s) |
|
 Analysis Tools
Analysis Tools